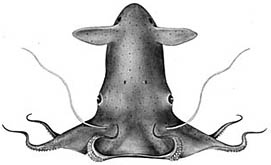
Vampyroteuthidae
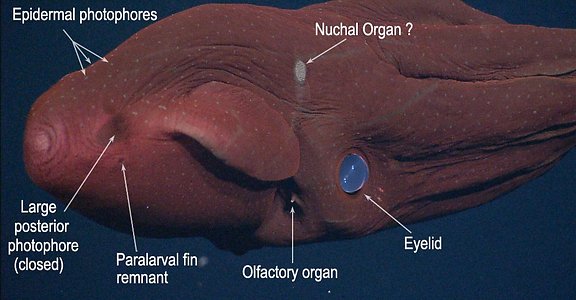
Vampyroteuthis infernalis is the only species known in the Vampyroteuthidae. The vampire squid is rather small, reaching a maximum of 21 cm ML (Hoving and Robison, 2012), and is very gelatinous; its consistency is that of a jellyfish. It occupies meso- to bathypelagic depths throughout the tropical and temperate regions of the world's oceans. The second pair of arms, apparently, is modified into retractile filaments that can extend to lengths up to eight times the total length of the animal, and they can be retracted into pockets between arms I and II (Hoving and Robison, 2012). The filaments appear to have a role in prey capture (Hunt, 1996), but in a novel fashion (see "Arms" next section). The vampire has black chromatophores with reddish-brown ones interspersed. [in most regions of the world's oceans, the black chromatophores dominate the general pigmentation, but off central California (e.g., the next two photographs) the reddish-brown cells dominate.] These chromatophores, however, have lost the muscles that enable the rapid color change seen in the chromatophores of other coleoids. A few normal coleoid chromatophores (i.e., chromatophore organs), however, are present on the vampire squid in the large, posterior photophores.
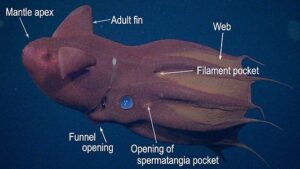
The vampire sqid is a phylogenetic relict and possesses features of both octopodiforms and decapodiformes (most phylogenetic studies place it as an early branch of the Octopodiformes). In addition, it has many features that are probably adaptations to the deep-sea environment. Among these latter are the loss of the ink sac and most chromatophores organs, the development of photophores and the gelatinous consistency of the tissues.
An octopodiform ...
- with long, slender filaments (probably modified arms II).
- with cirri but without suckers on the proximal halves of arms.
Vertical Distribution
Off California trawling data show most vampires between depths of 600-1100 m with peaks at 700-800 m and 900-1000 m, and with small individuals of less than 20 mm being most abundant at the deeper peak (Roper and Young, 1975). ROV observations in Monterey Bay, California suggest that the vampire occupies the oxygen minimum layer at depths of600-900 m with oxygen levels centered around 0.4 ml/l (Hoving and Robison, 2012).
Off Hawaii, 10 of 11 captures came from depths of 800-1200 m but little towing was done in deeper water. Two captures were from opening-closing nets at depths of about 800-950 m.
In the Atlantic at 18° N, 25° W, the vampire shows a peak distribution between 700 and 1200 m but without a clear size/depth pattern (Clarke and Lu, 1975).
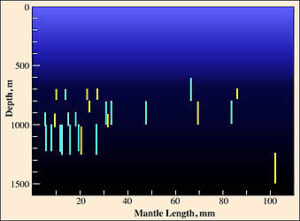
Figure. Vertical distribution chart modified from Clarke and Lu, 1975.
In the figure, all captures were made with opening/closing trawls. Bars represent a capture and the bar length indicates the depth range of the trawl while open. Yellow bars indicate a daytime capture and blue bars a nighttime capture. Fishing effort between 1000-1250 m was about twice that between 1250 and 1500 m, and effort between 1000-1500 m was about 5 times that between 1500 and 2000 m and about the same as that between 500 and 1000 m.
Numerous records exists for captures in excess of 1200 m (e. g., see Roper and Young, 1975) from open nets. Unfortunately, due to the rather high probability of contamination from shallower depths, these records are of questionable value.
Horizontal Distribution
The vampire squid is broadly distributed throughout the depths of the world's tropical and temperate oceans. Some geographical variation has been noted. Young (1972) found that the beaks of vampire squid from the Pacific Ocean off California were distinctly smaller than those from vampires of the Gulf of Guinea in the Atlantic Ocean. He also noted differences in sucker size and gill size in vampires from these areas. Vampires from off Monterey, California have a predominance of reddish rather than black chromatophores.
- Vampyromorpha (
- Vampyroteuthidae (
- Vampyroteuthis
- The Vampire Squid
Nominal Genus-Level Taxa:
Danateuthis Joubin, 1929:375.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis Chun, 1903 [fide Pickford (1946:7)]
- TYPE SPECIES. -- Danateuthis schmidti Joubin, 1929 by monotypy
Hansenoteuthis Joubin, 1929:388.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis Chun, 1903 [fide Nesis (1987:276)]
- TYPE SPECIES. -- Hansenoteuthis lucens Joubin, 1929 by monotypy
Hymenoteuthis Thiele, 1916:4.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis Chun, 1903 [fide Pickford (1946:7)]
- TYPE SPECIES. -- Cirroteuthis macrope Berry, 1911 by monotypy
Melanoteuthis Joubin, 1912:1, figs 1-12.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis Chun, 1910 [fide Pickford (1939:1)
- TYPE SPECIES. -- Melanoteuthis lucens Joubin, 1912 by monotypy
Retroteuthis Joubin, 1929:383.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis Chun, 1903 [fide Pickford (1946:7)]
- TYPE SPECIES. -- Retroteuthis pacifica Joubin, 1929 by monotypy
Vampyroteuthis Chun, 1903:88, text-fig.
- CURRENT SYSTEMATIC STATUS. -- Valid genus [fide Robson (1932:98)]
- TYPE SPECIES. -- Vampyroteuthis infernalis Chun, 1903 by monotypy
Watasella Sasaki, 1920:168.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis Chun, 1903 [fide Nesis (1987:276)]
- TYPE SPECIES. -- Watasella nigra Sasaki, 1920 by monotypy
Nominal Species-Level Taxa (As Introduced Binomial)
Melanoteuthis anderseni Joubin, 1931:170, figs 1-5.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis infernalis Chun, 1903 [fide Pickford (1946:7)]
- TYPE REPOSITORY. -- ZMUC Holotype [fide Kristensen and Knudsen (1983:220)] [see also Pickford (1949:124)]
- TYPE LOCALITY. -- 7°30'N, 79°19'W (Pacific Ocean)
Melanoteuthis beebei Robson, 1929:470, figs 1-8.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis infernalis Chun, 1903 [fide Pickford (1946:9)]
- TYPE REPOSITORY. -- BMNH Holotype 1947.5.21.1 [not 1947.7.7.3-6 fide Lipinski et al. (2000:110); incorrect catalog number, same as Helicocranchia beebei syntypes]
- TYPE LOCALITY. -- South of Coccos Island, 4°45'N, 87°00'W (Pacific Ocean)
Vampyroteuthis infernalis Chun, 1903:88, text-fig.
- CURRENT SYSTEMATIC STATUS. -- Valid species [fide Pickford (1939:48)]
- TYPE REPOSITORY. -- ZMB Holotype Moll.-63850 [fide Glaubrecht and Salcedo-Vargas (2000:275)]
- TYPE LOCALITY. -- 1°56.7'S, 7°40.6'E (Atlantic Ocean) [fide Glaubrecht and Salcedo-Vargas (2000:275)]
Hansenoteuthis lucens Joubin, 1929:388, figs 17-23.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis infernalis Chun, 1903 [fide Pickford (1946:7)]
- TYPE REPOSITORY. -- ZMUC Holotype [fide Kristensen and Knudsen (1983:222)] [see also Pickford (1949:123)]
- TYPE LOCALITY. -- 14°38'N, 61°16'W (Caribbean Sea)
Melanoteuthis lucens Joubin, 1912:1, figs 1-12.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis infernalis Chun, 1910 [fide Pickford (1939:1)
- TYPE REPOSITORY. -- MOM Holotype [station 2118] [fide Belloc (1950:2)]
- TYPE LOCALITY. -- Sargasso Sea, 31°40[']30"N, 42°44'30"W
Cirroteuthis macrope Berry, 1911:589.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis infernalis Chun, 1903 [fide Pickford (1946:7)]
- TYPE REPOSITORY. -- NMNH Holotype 214317 [fide Sweeney et al. (1988:15)] [see also Pickford (1949:127)]
- TYPE LOCALITY. -- 32°54'20"N, 121°11'15"W (Pacific Ocean)
Watasella nigra Sasaki, 1920:168, pl 23 fig 1.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis infernalis Chun, 1903 [fide Pickford (1946:9)]
- TYPE REPOSITORY. -- NMNH Holotype 332892 [fide Roper and Sweeney (1978:10)] [see also Pickford (1949:128)]
- TYPE LOCALITY. -- Off Kii Province (33°23'N, 135°37'40"E), Japan
Retroteuthis pacifica Joubin, 1929:383, figs 13-16.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis infernalis Chun, 1903 [fide Pickford (1946:7)]
- TYPE REPOSITORY. -- ZMUC Holotype [fide Kristensen and Knudsen (1983:223)] [see also Pickford (1949:126)]
- TYPE LOCALITY. -- 7°30'N, 79°19'W (Pacific Ocean)
Danateuthis schmidti Joubin, 1929:375, figs 7-12.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis infernalis Chun, 1903 [fide Pickford (1946:7)]
- TYPE REPOSITORY. -- ZMUC Holotype [fide Kristensen and Knudsen (1983:224)] [see also Pickford (1949:122)]
- TYPE LOCALITY. -- 18°50'N, 79°07'W (Caribbean Sea)
Melanoteuthis schmidti Joubin, 1929:372, figs 3-6.
- CURRENT SYSTEMATIC STATUS. -- Vampyroteuthis infernalis Chun, 1906 [fide Pickford (1946)]
- TYPE REPOSITORY. -- ZMUC Holotype [fide Kristensen and Knudsen (1983:224)] [see also Pickford (1949:126)]
- TYPE LOCALITY. -- 17°56'N, 64°50'W (Caribbean Sea)
Type Repositories
- BMNH-The Natural History Museum, Cromwell Road, London SW7 5BD, England (formerly British Museum (Natural History)). [For type catalog see Lipinski et al. (2000)]
- MOM- Musée Océanographique, Avenue Saint-Martin, Monaco-Ville, MC 98000, Monaco. [For type catalog see Belloc (1950)]
- NMNH-National Museum of Natural History, 10th & Constitution Avenue, Smithsonian Institution, Washington, DC 20560, U.S.A. (formerly USNM). [For type catalogs see Roper and Sweeney (1978); Sweeney et al. (1988)]
- ZMB- Zoologisches Museum, Museum für Naturkunde der Humboldt-Universitat, Invalidenstrasse 43, D-1040 Berlin, Germany. [For type catalog see Glaubrecht and Salcedo-Vargas (2000)]
- ZMUC-Kobenhavns Universitet, Zoologisk Museum, Universitetsparken 15, DK 2100 Copenhagen, Denmark. [For type catalog see Kristensen and Knudsen (1983); See also Volsoe et al. (1962) for Steenstrup papers]
- Arms
- Arms II (probably) modified to form retractile filaments (see details below).
- Lateral cirri present over arm length; suckers present only on distal half of arms where they alternate with cirri.
- Suckers without cuticular lining; sucker stalks constricted distally to narrow plate at base of sucker.

Figure. Ventro-oral view of V. infernalis with arms folded aborally, showing cirri without associated suckers in proximal half of arms. Note the predominance of black pigment, compared to the previous two photographs, and the colorless, oral tips of the arms. Photographed in a shipboard aquarium in the North Atlantic by David Shale.
- Retractile filaments
- Origin of the filaments:
- The primitive (plesiomorphic) arrangement of arms in coleoid cephalopods is thought to be 10 equal arms. This arrangement is known in some fossil coleoids (belemnoids) and the presence of ten unequal arms in modern decapods is easily derived from such a condition. Therefore, octopods, with eight arms, have apparently lost one pair. If the vampire filaments represent modified arms II and if this is the pair that is lacking in octopods, then strong support would exist for a vampire-octopod affinity. Embryological evidence suggests that the missing arm pair in octopods is either arms II or III (Boletzky, 1978-79).
- Pickford (e.g., 1940) concluded that the filaments were modified arms II based on their position in series with the arms, venous connections and innervation. Young (1967) clarified the innervation and argued that the filaments were not arms but homologues of the preocular tentacles of Nautilus. The axial nerves of arms send most of their fibers to the anterior subesophageal mass (brachial lobe) of the brain. Young found that the filament nerves bypass this lobe with efferent fibers arising from the middle subesophageal mass and the afferent fibers reaching an enlarged ventral magnocellular lobe. Dilly, et al. (1977), however, demonstated that large nerve bundles from the tentacles of the deep-sea squid Mastigoteuthis also pass to an enlarged ventral magnocellular lobe. Presumably the combined sensory function of thousands of suckers carried by the tentacles of Mastigoteuthis requires the large nerve bundle and the enlarged ventral magnocellular lobe. Similarly the still poorly understood functions of the filaments of Vampyroteuthis probably emphasized the connection to and size of the ventral magnocellular lobe while a loss of coordination with the principal arms resulted in a great reduction (at least) in the connection to the anterior subesophageal mass. As a result, the innervation of the filaments became a weak argument against filament-arm homology.
- The homology of the filament appeared to be resolved when J. Z. Young (1977) reported a connection between the axial nerve of the vampire filament and ganglia of the circumoral commissure that connects the axial nerves of all arms. This evidence, however, could not be confirmed by Young and Vecchione (1996).
- Recent confirmation of Pickford's earlier evidence (Pickford, 1946) that the vampire hatchling has relatively thick (i.e., more arm-like) filaments supports the homology between arms and filaments (Young and Vecchione, 1999)
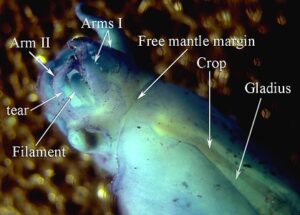
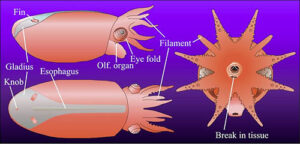
Figure. Vampyroteuthis infernalis hatchling. Figures modified from Young and Vecchione, 1999. Top - Dorsal and slightly lateral photograph of the lightly stained (methylene blue) hatchling. Bottom - Lateral, dorsal and anterior drawings of the same hatchling. Morphologically the specimen is an advanced embryo with a large internal yolk sac. - The weight of evidence indicates that the filaments represent arms II. Proof may depend on examining the embryonic development of the vampire squid which is not feasible at present.
- The filament:
- Each long filament has muscles that run the along one side. Contraction of these muscles causes coiling of the filament and its retraction into the small filament pocket that lies at the base of the web. The following images show a filament that has been removed from a recently dead vampire. Most of the filament has an orange pigmentation; the cut base (arrow), however, is abruptly broader and has a pink color. Much of the tight coiling has been disrupted by handling.
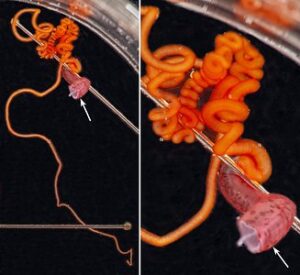
Figure. Filament of Vampyroteuthis infernalis. Left - The entire filament from the cut base (arrow) to the filament tip. Right - Enlargement of the upper part of the left image showing how tight some of the undisturbed coils can be. Photographed in a small dish aboard ship by Henk-Jan Hoving. - Under the dissecting microscope numerous, straight "hairs" are seen extending from the filament. These "hairs" are thought to aid in capturing food.
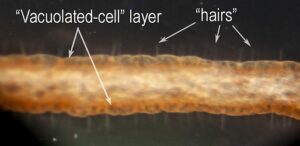
Figure. V. infernalis filament. Photomicrograph of a portion of the intact, fresh filament. Photograph by H. Hoving. - The Histology of the filaments has been examined by Hoving and Robison, 2012. The "hairs" could not be detected in the paraffin-embedded sections but could be seen in SEM photographs collapsed onto the surface of the filament; there, they appear to be tree-like branching structures rather than simple hairs. The surface of the filament consists of a layer of "vacuolated" cells with little internal structure. In SEM photographs, these cells are also collapsed and the "hairs" seem to arise at the junctures between these cells. Beneath the vacuolated cell layer is a layer of simple cuboidal cells with larger cells broadly interspersed. These larger cells are apparently nerve cells as they have extensions that pass into a large, axial nerve. A large muscle exists along one side of the filament and its contraction results in the coiling of the filament.
- The "hairs" apparently secure food particles (Hoving and Robison, 2012). The retraction of the filaments is a slow process and the vampire squid may swim to the portion of the tentacle holding the food (Hunt, 1996) and enclose it within the web. Next, the filament is drawn between the arms, wrapped in mucous produced by cells on the suckers, sucker stalks and cirri, and passed to the mouth (Hoving and Robison, 2012).
- Each long filament has muscles that run the along one side. Contraction of these muscles causes coiling of the filament and its retraction into the small filament pocket that lies at the base of the web. The following images show a filament that has been removed from a recently dead vampire. Most of the filament has an orange pigmentation; the cut base (arrow), however, is abruptly broader and has a pink color. Much of the tight coiling has been disrupted by handling.
- Origin of the filaments:
- Head
- Eyelid circular, without anterior optic sinus.
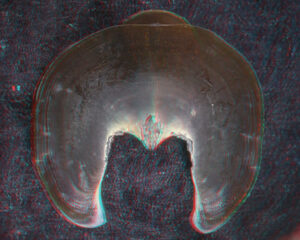
- Beaks:
- Lower beak:
V. infernalis, sex ?, 13 mm GL, 1.0 mm LRL, Hawaiian waters. Photographs by R. Young V. infernalis, sex ?, ~40 mm ML, 2.2 mm LRL, Hawaiian waters. Photographs by R. Young. V. infernalis, sex ?, ML ?, 5.3 mm LRL, Hawaiian waters. Photographs by R. Young. Side View 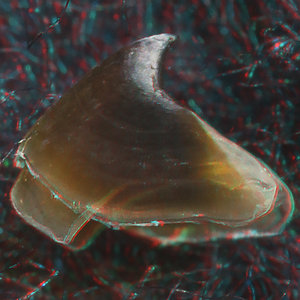


Oblique View 


Oblique View 


“Top” view 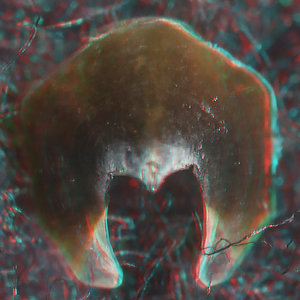

Oral view 


Front View 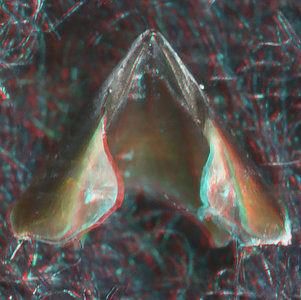
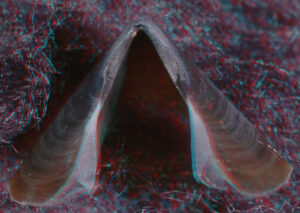

Posterior Oblique View 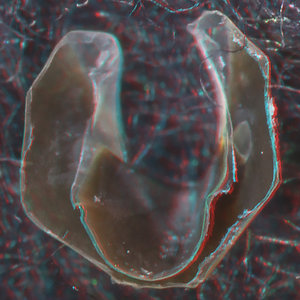

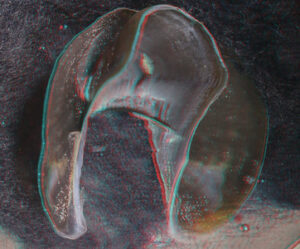
- About anaglyph 3D: Photographs here are in 3D, using anaglyph techniques which combines two photographs one in red and the other in cyan (blue + green) color. To get the 3D effect, you must use glasses with red and cyan lenses (filters) over your regular glasses. The form of the beak is far easier to interpret in 3D and we strongly recommend to the viewer that the glasses be obtained (this is especially helpful when viewing enlarged images - i.e. click on the image). These beak photographs were made using Red and Cyan Anachrome Aviator Glasses (see: http://www.anachrome.com/glassbuy.htm or http://www.amazon.com) which cost under $10.00 (USD).
- Upper beak:
V. infernalis, sex ?, 13 mm GL, 1.0 mm LRL, Hawaiian waters. Photographs by R. Young V. infernalis, sex ?, ~40 mm ML, 2.2 mm LRL, Hawaiian waters. Photographs by R. Young. V. infernalis, sex ?, ML ?, 5.3 mm LRL, Hawaiian waters. Photographs by R. Young. Side View 


Oblique View 


Oblique View 


“Top” view 

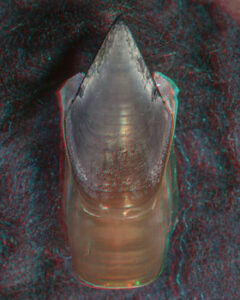
Oral view 


Front View 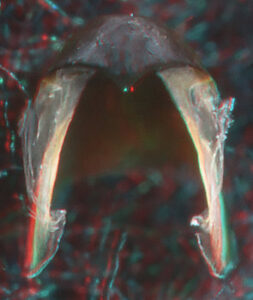

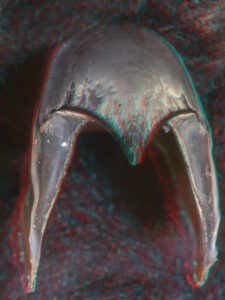
Posterior Oblique View 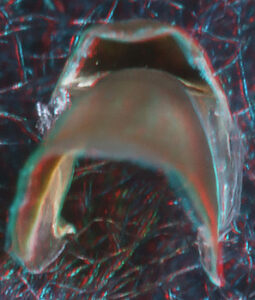


- Lower beak:
- Nuchal cartilage present even though head and mantle broadly fused.
- Central nervous system with incipient inferior frontal lobe system; superior buccal lobes adjacent (fused at edges) to posterior buccal lobes; suprabrachial commissure lies within brain.
- Funnel
- Funnel valve absent.
- Mantle
- Dorsal mantle cavity absent.
- Mantle apex a highly-flexible muscular cone filled with gelatinous tissue located posterior to the cone of the gladius.
- Fins
- Two pairs of fins present during early ontogeny.
- Spermatangia
- Receptacle (deep sac) for spermatangia located anterior to each eye in females.
- Shell
- Shell a gladius with broad median field and broad conus with or without a short, narrow cyclindrical rostrum.
- Photophores
- Large circular, lidded organs (= "fin-base" organs or "large posterior photophores") present posterior to each adult fin .
- Numerous small organs distributed over ventral surfaces of mantle, funnel, head and aboral surface of arms and web (="skin-nodule" organs or "epidermal photophores"). Two patches on dorsal surface of head look like aggregrated small photophores (see images in Introduction) but are photoreceptors (Herring, et al., 1994) and may be "Nuchal Organs" (see Parry, 2000).
- Arm-tip organ(s) produce luminescent clouds consisting of microscopic glowing particles (Robison, et al., 2003).
- Arm-tip organs that flash or glow (Hunt, 1996).
- Pigmentation
- Photographs, of the vampire squid, taken in situ at two different locations show the geographic differences in coloration:

Figure. Vampyroteuthis infernalis. Left - Side view, central North Pacific. Right - Oblique-anterior view, Monterey Bay. Note the difference in color between the two. © MBARI - These photographs of Vampyroteuthis were taken in situ by an ROV of the Monterey Bay Aquarium Research Institute. The two photographs were taken from widely different areas in the North Pacific showing geographic differences in coloration. The left image is a side view of the vampire in an unusual pose. Note the peculiar nature of the web in this posture which seems to suggest how the web can contract and expand. Also peculiar are the small epidermal photophores on the arms that seem to align in series. In the right image, the arms are aligned and the presence of a web is hardly noticed.
- Photographs, of the vampire squid, taken in situ at two different locations show the geographic differences in coloration:
- Viscera
- Oviducal glands at terminal end of oviducts.
- Visceropericardial coelom extends posteriorly as a slender duct, to a small organ at the apex of the conus of the gladius. This duct is possibly a remnant siphuncle.
- Photosensitive vesicles located immediately dorsal to funnel and, possibly, on dorsal integument in nuchal region.
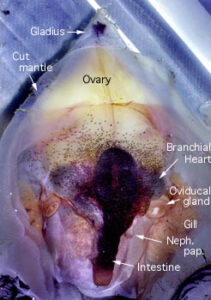
Comments: Mantle Cavity
Ventral view of an immature, female V. infernalis with the mantle cavity of cut open. Posteriorly the lining of the mantle cavity has been cut away to better view the visceral nucleus. Note that there is no abdominal septum, no ink sac, no anal flaps. The visceropericardial coelom (dark region surrounding the ventricle, stomach and caecum) is large. Posteriorly, in the ventral midline, the mantle muscle terminates on the conus of the gladius. The photograph on the right shows a mature female. The arrangement of the viscera looks very different. The gladius, with a small rostrum and a dark "apical organ", is visible because surrounding tissue has been removed.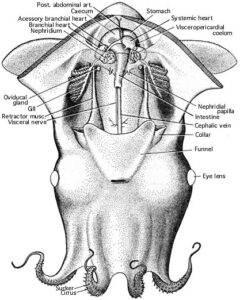

- Fins
- Development of the fins in the vampire is unique among cephalopods. One pair is present at hatching and, with only a slight increase in mantle length (if any), a second, more anterior fin-pair (the adult fins) develops giving the vampire squid four swimming fins. At about 25 mm ML the hatchling fins are quickly resorbed (Pickford, 1949). The first fin-pair to develop is the homologue of the fins of other cephalopods (Young and Vecchione, 1996). The unusual fin ontogeny is partially responsible for the early description of 3 families and many species where only one species actually exists. Except for the fins, the young vampire squid (about 10 mm ML) have an appearance very similar to that of the adult.

Figure. The photograph shows an advanced four-fin stage in which the anterior, adult fins are much larger than the posterior hatchling-fins which will soon be resorbed. Note the mm scale, and the open large, posterior photophores. Photograph by John Bower.
- Development of the fins in the vampire is unique among cephalopods. One pair is present at hatching and, with only a slight increase in mantle length (if any), a second, more anterior fin-pair (the adult fins) develops giving the vampire squid four swimming fins. At about 25 mm ML the hatchling fins are quickly resorbed (Pickford, 1949). The first fin-pair to develop is the homologue of the fins of other cephalopods (Young and Vecchione, 1996). The unusual fin ontogeny is partially responsible for the early description of 3 families and many species where only one species actually exists. Except for the fins, the young vampire squid (about 10 mm ML) have an appearance very similar to that of the adult.
- Eggs and hatchlings
- Vampires lack nidamental glands and have rather small oviducal glands. As a result there is little likelihood that they produce large egg masses. Off California small vampire squid occupy greater depths than do the larger individuals (Roper and Young, 1975) suggesting that spawning occurs in very deep water.
- A recently hatched Vampyroteuthis (8 mm ML) looks very different in shape, color and general proportions from the paralarva or subadult. The latter two look quite similar except for the different fins present and the smaller size of the eyes in paralarvae. The hatchling, however, differs in having short arms, no web, no dark pigment, no superficial gelatinous layer covering the head and mantle, no fusion of the head and mantle and a narrower gladius in the anterior half. A large amount of yolk can be seen internally through the transparent gladius. The filaments have a uniform diameter throughout, are short and thick, and arise in series with the eight normal arms.
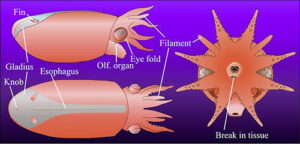
Figure. V. infernalis hatchling, 8 mm ML, captured off Hawaii, 1050-1300 m depth. Drawing modified from Young. and Vecchione, 1999. - The following photograph was made with transmitted light on the newly captured (i.e., unfixed) hatchling. The dark line is a pin placed on the hatchling to keep it from rolling, or floating while being photographed.
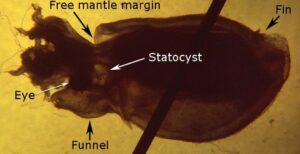
Figure. Lateral view with slight dorsal tilt of V. infernalis, hatchling. Photograph with transmitted light modified from Young. and Vecchione, 1999. - This next photograph was made with reflected light on the preserved and stained (methylene blue) hatchling. The esophagus expands slightly forming a crop.
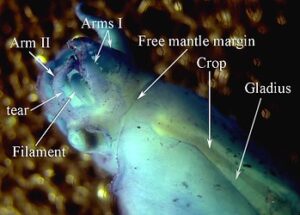
Figure. Reflected light photograph of the V. infernalis hatchling. Photograph modified from Young and Vecchione, 1999. - Either this specimen hatched prematurely due to the trauma of its capture in a trawl, or it indicates that the hatchlings drift in deep water until the yolk is utilized and they then transform into the typical Vampyroteuthis form. The size of the hatchling suggests that it came from an egg of nearly 8 mm in diameter. Pickford (1949) identified 4 mm eggs from plankton tows as belonging to Vampyroteuthis. This discrepancy cannot be explained at present.
Behavior
Vampyroteuthis can swim surprisingly fast for a gelatinous animal. Hunt (1996) estimates from videotapes that it can reach two body lengths/sec, and it can accelerate to this speed in 5 sec. An escape reaction involves the quick movement of the fins toward the funnel followed by a jet from the mantle. This sequence is repeated as the vampire takes a series of quick turns in an erratic escape route (Hunt, 1996).




Postures:
In slow swimming, the arms are sometimes spread anteriorly to form, along with the web, the shape of an umbrella or bell while the vampire slowly swims forward (Hunt, 1996). In another posture ("pineapple posture"), the arms and web are spread aborally over the head and mantle (Robison, 1995). In this defensive posture the squid would be somewhat more difficult to injure and would be covered by a densely pigmented cloak. The oral surface of the arms and webs are the most heavily pigmented (black) regions on the animal.
Bioluminescence:
Hunt (1966) first observed bioluminescence displays in the living animal. The two large posterior photophores have been observed to glow brightly for less than a second (a flash) or longer than two minutes. The light intensity can vary giving a pulsating appearance, and, in addition, as light is extinguished, the glowing disc can be seen to decrease in diameter as well as intensity.

Arm-tips are unpigmented on their oral surface allowing light to be emitted from surfaces that have no obvious luminescent structures (see photograph on the right). These surfaces can glow, or they can flash at a rate of one to three per second or pulsate. With the arm-tip organs apparently glowing continuously, the vampire moves the arms ("arm writhing") around rapidly alternately exposing and hiding the luminescence which is "...very disorienting [to an observer] when trying to visually fix the animal's position" (Hunt, 1996 p. 104). Often a flash of the arm tips is followed by a rapid escape response. Another unusual and visually confusing effect is seen when viewing the vampire posteriorly (i.e., looking toward the mantle apex). The apparently disturbed vampire can curl the arms and web posteriorly over the head ("pineapple posture") then illuminate the arm-tip organs and the large posterior photophores. Hunt (1996, p. 104) states that the arm tips appear to come toward you, whereas the large posterior photophores appear to be moving away (due to their internal contraction) .
A third source of bioluminescence is the production of luminescent clouds whose function, seemingly, is to startle or distract a predator. These clouds appear as a mucous matrix with a few hundred to over 1000 discrete, glowing particles embedded in it. The particles can glow for up to 9.5 min. The source of the particles has been shown to be the arm tip organs (Robison, et al., 2003). The latter authors also suggest that flashing of the arm-tip organs is controlled by covering or exposing the photogenic region by the pigmented sides of the arm tips, and that the microscopic glowing particles of the luminescent clouds, are not luminescent bacteria.
Feeding:
The vampire appears to orient most commonly in a horizontal attitude with generally one filament extended (Hunt, 1996). laboratory observations on living vampires suggest the filaments are tactile sense organs (Hunt, 1996) used in detecting prey. Hoving and Robison (2012) found that the filaments, also, aid in "capturing" prey which is primarily detritus.
Berry, S.S. 1911. Preliminary notices of some new Pacific cephalopods. Proceedings of the United States National Museum, 40(1838):589-592. Belloc, G. 1950. Catalogue des types de céphalopodes du Musée Océanographique de Monaco. Bulletin de l'Institut Océanographique, 970:1-10.
Boletzky, S.v. 1978/79. Nos connaissances actuelles sur le développement des Octopodes. Vie et Milieu, 28-29(1AB):85-120.
Chun, C. 1903. Aus den Tiefen des Weltmeeres. 592 pages. Jena: Gustav Fischer, second edition.
Chun, C. 1915. Die Cephalopoden. Myopsida, Octopoda. Wissebschaftliche ergebnisse der deutschen tiefsee expedition auf dem dampfer "Valdivia" 1898-1899, 18(2):405-552, 414 figures, 34 plates.
Clarke, M. R. and C. C. Lu. 1975. Verical Distribution of Cephalopods at 18° N 25° W in the North Atlantic. Journal of the Marine Biological Association of the United Kingdom, 55 (1): 165-182.
Glaubrecht, M. and M.A. Salcedo-Vargas. 2000. Annotated type catalogue of the Cephalopoda (Mollusca) in the Museum fur Naturkunde, Humboldt University of Berlin. Mitteilungen aus dem Museum fur Naturkunde Berlin, Zoologischen, 76(2):269-282.
Herring, P. J., P. N. Dilly and C. Cope 1994. The bioluminescent organs of the deep-sea cephalopod Vampyroteuthis infernalis (Cephalopoda: Vampyromorpha). J. Zool. Lond. 233: 45-55.
Hunt, J. C. 1996. The behavior and ecology of midwater cephalopods from Monterey Bay: Submersible and laboratory observations. Ph. D. Dissertation, Univ. Calif. Los Angeles. 231 pp.
Hendrik J. T. Hoving and Bruce H. Robison. 2012. Vampire squid: detritivores in the oxygen minimum zone. Proc. R. Soc. B published online 26 September 2012 doi: 10.1098/rspb.2012.1357
Joubin, L. 1912. Etude preliminaires sur les Céphalopodes recuillis au cours des croisières le S.A.S. le Prince de Monaco. 1re Note: Melanoteuthis lucens nov. gen. et sp. Bulletin de l'Institut Océanographique, 220:1-14, 12 figures.
Joubin, L. 1929. Notes preliminaires sur les Céphalopodes des croisières du DANA (1921-1922). Octopodes - 1re Partie. Annals de l'Institut Océanographique, (new series)6(4):363-394, 23 figures.
Joubin, L. 1931. Notes préliminaires sur les Céphalopodes des croisières du DANA (1921-1922), 3e Partie. Annals de l'Institut Océanographique, (new series)10(7):169-211, 48 figures.
Kristensen, T.K. and J. Knudsen. 1983. A catalogue of the type specimens of Cephalopoda (Mollusca) in the Zoological Museum, University of Copenhagen. Steenstrupia, 9(10):217-227.
Lipinski, M.R., F.A. Naggs, and M.A. Roeleveld. 2000. Catalogue of types of Recent cephalopods in the collection of the Natural History Museum, London. Annales Zoologici (Warszawa), 50(1):101-120.
Nesis, K. N. 1982. Abridged key to the cephalopod mollusks of the world's ocean. 385,ii pp. Light and Food Industry Publishing House, Moscow. (In Russian.). Translated into English by B. S. Levitov, ed. by L. A. Burgess (1987), Cephalopods of the world. T. F. H. Publications, Neptune City, NJ, 351pp.
Nesis, K.N. 1987. Cephalopods of the World; Squids, cuttlefishes, Octopuses, and Allies. T.F.H. Publications, Neptune City, NJ, USA, 351 pages.
Parry, M. 2000. A description of the nuchal organ, a possible photoreceptor, in Euprymna scolopes and other cephalopods. J. Zool. Lond. 252: 163-177.
Pickford, G.E. 1936. A new order of dibranchiate Cephalopoda. The Anatomical Record, 67(1)(supplement 1):77-78.
Pickford, G.E. 1939. A re-examination of the types of Melanoteuthis lucens Joubin. Bulletin de l'Institut Oceanographique, 777:1-12.
Pickford, G.E. 1940. The Vampyromorpha, living-fossil cephalopoda. Transactions of the New York Academy of Sciences, (ser. 2) 2(7):169-181.
Pickford, G.E. 1946. Vampyroteuthis infernalis Chun an archaic dibranchiate cephalopod. I. Natural history and distribution. Dana Report, 29:1-40.
Pickford, G. E. 1949. Vampyroteuthis infernalis Chun an archaic dibranchiate cephalopod. II. External anatomy. Dana-Report No. 32: 1-132.
Pickford, G. E. 1949. The distribution of the eggs of Vampyroteuthis infernalis Chun. Journal of Marine Research, 8(1):73-83.
Robson, G.C. 1929. On the rare abyssal octopod Melanoteuthis beebei (sp. n.): a contribution to the phylogeny of the Octopoda. Proceedings of the Zoological Society of London, 1929(3):469-486, 8 figures.
Robson, G.C. 1932. A Monograph of the Recent Cephalopoda. Part II. The Octopoda. 359 pages, 79 figures, 6 plates. London: British Museum.
Robison, B. H. 1995. Light in the ocean's midwaters. Scientific American 273:60-64.
Robison, B. H, K. R. Reisenbichler, J. C. Hunt and S. H. D. Haddock. 2003. Light production by the arm tips of the deep-sea cephalopod Vampyroteuthis infernalis. Biol. Bull., 205: 102-109.
Roper, C. F. E. and R. E. Young. 1975. Vertical distribution of pelagic cephalopods. Smithsonian Contributions to Zoolog, 209: 1-51.
Roper, C.F.E. and M.J. Sweeney. 1978. A catalog of the type-specimens of Recent Cephalopoda in the National Museum of Natural History. Smithsonian Contributions to Zoology, 278:1-19.
Sasaki, M. 1920. Report on cephalopods collected during 1906 by the United States Bureau of Fisheries steamer "Albatross" in the northwestern Pacific. Proceedings of the United States National Museum, 57(2310):163-203, 4 plates.
Seibel, B. A., Thuesen, E. V. and Childress, J. J. 1998. Flight of the Vampire: Ontogenetic gait-transition in Vampyroteuthis infernalis (Cephalopoda: Vampyromorpha). J. exp. Biol. 201, 2413-2424.
Sweeney, M.J., C.F.E. Roper, and F.G. Hochberg. 1988. Catalog of the type specimens of Recent Cephalopoda described by S. Stillman Berry. Malacologia, 29(1):7-19.
Thiele, J. 1916. Bemerkungen über die Systematik der achtarmigen Cephalopoden. Zoologischer Anzeiger, 48(1):3-4.
Volsoe, A., J. Knudsen and W. Rees. 1962. The cephalopod papers of Japetus Steenstrup; a translation into English. Danish Science Press, Copenhagen, 330 pages.
Young, J. Z. 1977. Brain, Behaviour and Evolution of Cephalopods. Symp. Zool. Soc., London, 38: 377-434.
Young, R. E. 1964. The anatomy of the vampire squid. Masters Thesis, Univ. of Southern Calif.
Young, R.E. 1967. Homology of retractile filaments of vampire squid. Science, 156(3782):1633-1634.
Young, R. E. 1972. The systematics and areal distribution of pelagic cephalopods from the seas off Southern California. Smithson. Contr. Zool., 97: 1-159.
Young, R. E. and M. Vecchione 1996. Analysis of morphology to determine primary sister-taxon relationships within coleoid cephalopods. Am. malacol. Bull. 12: 91-112.
Young, R. E., and M. Vecchione. 1999. Morphological observations on a hatchling and a paralarva of the vampire squid, Vampyroteuthis infernalis Chun (Mollusca: Cephalopoda). Proc. Biol. Soc. Wash., 112: 661-666.